Product Specifications
| Specifications | Parameters |
|---|---|
| Dye Content (By Spectrophotometry) | >70% |
| Transition Range | Ph 4.3-6.3 |
| Yellow-Pink | |
| Ph 9.4-12.0 | |
| Brown Orange-Violet | |
| Solubility 0.1% (Dist. Water) | Clear Solution |
| Absorption Max (0.1N Naoh) Λ₁max | 553-559Nm |
| Absorption Max (0.1N Naoh) Λ₂max | 553-559Nm |
| Absorptivity (A1%/1Cm At 556 Λ₁max) | >350 |
| Absorptivity (A1%/1Cm At 595 Λ₂max) | >325 |
| Loss On Drying (110⁰C) | <5% |
| Suitability For Microscopy | Passes Test |
| Specifications | Parameters |
|---|
| C.A.S. No. | 76058-33-8 |
| Molecular Weight | 297.35g/mole |
| Solubility 0.1% (Ethanol) | Clear solution |
| Transition range | pH 4.2 – 6.5 |
| Red – Yellow | |
| Absorption maximum (pH 4.2) | 448 – 452nm |
| Absorptivity (1%/1cm at pH 4.2) | >1300 |
| Absorption maximum (pH 6.5) | 525-529nm |
| Absorptivity (1%/1cm at pH 6.5) | >700 |
| Loss On Drying at 110 degrees C (1hr) | < 3% |
Average Stock Availability (in Kg)
- Upto 25
- 25 to 100
- 100 to 500
- Above 1000
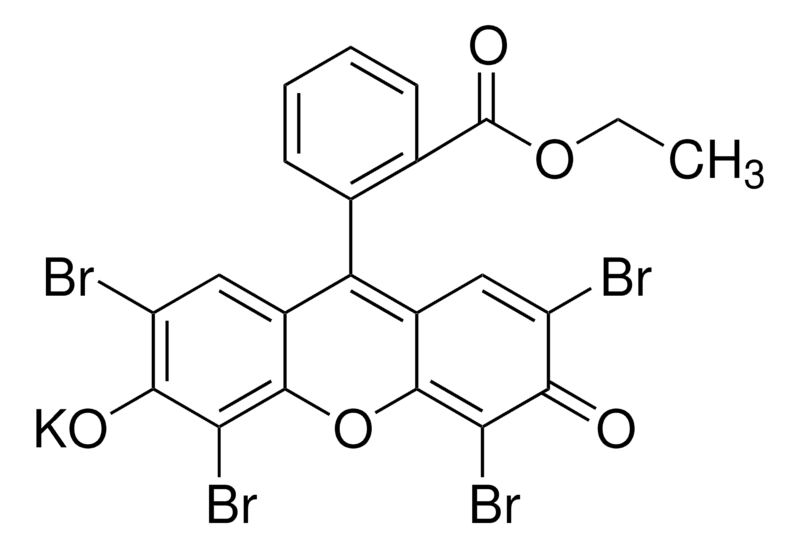
Ethyl Red
Ethyl red is used as a test compound in analytical chemistry to determine the presence of acidic or basic compounds in a solution. Major applications of Ethyl red pertain to Optical materials, photoresists, flexible electronic circuitry, adhesives, textiles, method for counting leukocytes, enzyme binding assays, DNA chips. Ethyl red is commonly used in analytical chemistry for the qualitative and quantitative determination of acids and bases, particularly in educational settings and routine laboratory procedures. It can be employed in organic chemistry research to monitor the progress of reactions involving changes in pH, such as hydrolysis and neutralization reactions. Ethyl Red is primarily used in microbiology, biochemistry, and analytical chemistry applications.
Our Product Category
faq's
frequently asked questions
ontrary to popular belief, Lorem Ipsum is not simply random text. It has roots in a piece of classical Latin literature from 45 BC, making it over 2000 years old. Richard McClintock, a Latin professor at Hampden-Sydney College in Virginia, looked up one of the more obscure Latin words, consectetur, from a Lorem Ipsum passage, and going through the cites of the word in classical literature, discovered the undoubtable source. Lorem Ipsum comes from sections 1.10.32 and 1.10.33 of “de Finibus Bonorum
ontrary to popular belief, Lorem Ipsum is not simply random text. It has roots in a piece of classical Latin literature from 45 BC, making it over 2000 years old. Richard McClintock, a Latin professor at Hampden-Sydney College in Virginia, looked up one of the more obscure Latin words, consectetur, from a Lorem Ipsum passage, and going through the cites of the word in classical literature, discovered the undoubtable source. Lorem Ipsum comes from sections 1.10.32 and 1.10.33 of “de Finibus Bonorum
ontrary to popular belief, Lorem Ipsum is not simply random text. It has roots in a piece of classical Latin literature from 45 BC, making it over 2000 years old. Richard McClintock, a Latin professor at Hampden-Sydney College in Virginia, looked up one of the more obscure Latin words, consectetur, from a Lorem Ipsum passage, and going through the cites of the word in classical literature, discovered the undoubtable source. Lorem Ipsum comes from sections 1.10.32 and 1.10.33 of “de Finibus Bonorum
ontrary to popular belief, Lorem Ipsum is not simply random text. It has roots in a piece of classical Latin literature from 45 BC, making it over 2000 years old. Richard McClintock, a Latin professor at Hampden-Sydney College in Virginia, looked up one of the more obscure Latin words, consectetur, from a Lorem Ipsum passage, and going through the cites of the word in classical literature, discovered the undoubtable source. Lorem Ipsum comes from sections 1.10.32 and 1.10.33 of “de Finibus Bonorum




